Research summary
Research in Nguyen group focuses on understanding chemical processes and applying that knowledge to improve them for industrial applications. The current main research areas in the group are:
1. Machine Learning methodologies to generate intepretable prediciton models chemical properties, reactivity and reaction outcomes, including impurities and workup.
2. High throughput computational and analytical techniques to generate high quality data for AI/Machine Learning in chemical sciences.
3. Theoretical and practical framework for discovery and development of 'on water' reactions as green processes in High Value Chemical Manufacture.
Our research tools include AI/Machine Learning and computational chemistry, structure-reactivity relationships, spectroscopic studies, kinetic analysis and models, and electrochemical chemistry. We are also part of the iPRD (https://www.iprd.leeds.ac.uk), and have access to a wide range of equipment and expertise in batch and flow reactors from lab scale to pilot scale.
Some of our recent projects are described below:
Accurate solubility prediction in organic solvent and aqueous media
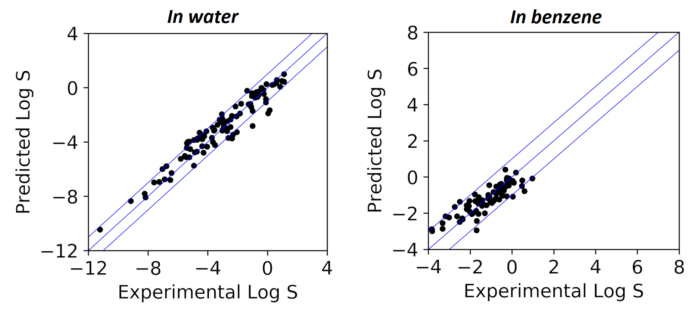 Solubility prediction remains a critical challenge in drug development, synthetic route and chemical process design, extraction and crystallisation. We developed a successful approach to solubility prediction in organic solvents and water using a combination of machine learning and computational chemistry. Rational interpretation of dissolution process into a numerical problem led to a small set of selected descriptors and subsequent predictions which are independent of the applied machine learning method. These models gave significantly more accurate predictions compared to benchmarked open-access and commercial tools, achieving accuracy close to the expected level of noise in training data (LogS±0.7). Finally, they reproduced physicochemical relationships between solubility and molecular properties in different solvents, which led to rational approaches to improve the accuracy of each models.
Solubility prediction remains a critical challenge in drug development, synthetic route and chemical process design, extraction and crystallisation. We developed a successful approach to solubility prediction in organic solvents and water using a combination of machine learning and computational chemistry. Rational interpretation of dissolution process into a numerical problem led to a small set of selected descriptors and subsequent predictions which are independent of the applied machine learning method. These models gave significantly more accurate predictions compared to benchmarked open-access and commercial tools, achieving accuracy close to the expected level of noise in training data (LogS±0.7). Finally, they reproduced physicochemical relationships between solubility and molecular properties in different solvents, which led to rational approaches to improve the accuracy of each models.
Catalyst development for industrial applications
X-ray Absorption Fine Structure Spectroscopy (XAFS) is a synchrotron-based spectroscopic technique which has recently found more and more applications in homogeneous systems. It offers unparalleled insights into the nature of transition metal intermediates under realistic catalytic conditions.
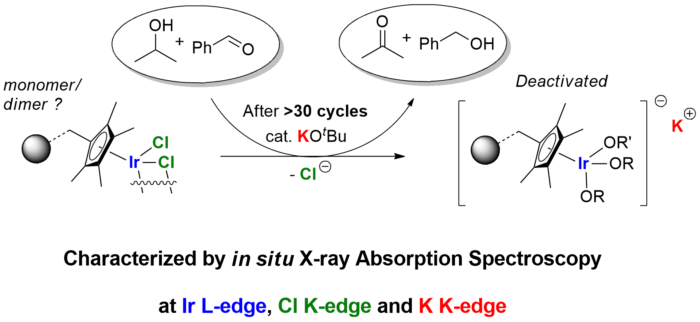
We recently reported (doi: 10.1021/om300030e) a mechanistic investigation into the deactivation of a robust immobilised catalyst for transfer hydrogenation from Yorkshire Process Technology. Immobilisation is an important strategy in converting synthetic catalysts in academia, which are often expensive, into recyclable catalysts for industrial applications. Common deactivation pathways such as metal leaching and ligand exchange, however, often cause immobilised catalysts to lose their catalytic activity much more quickly than desired. In this study, a highly novel combination of XAFS at Ir L-edge, Cl K-edge and K K-edge led to a complete characterisation of the deactivated catalyst, which was caused by ligand exchange between chlorides and alkoxides over time. Strategy to reactivate the catalyst and suppress catalyst deactivation was subsequently developed based on these insights.
Electrochemical flow-cell for production of catalysts on-demand
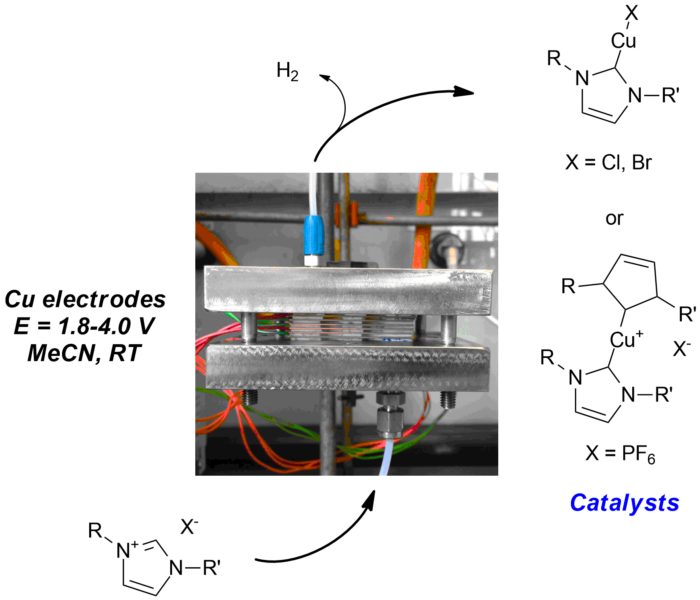 Through collaborations with other research groups in fluid dynamics and inorganic chemistry, we developed an extremely efficient and scalable electrochemical flow-cell which can effect the conversion of imidazoliums and a copper electrode to Cu-NHC catalysts (NHC = N-heterocyclic carbene) in very high yields (DOI: 10.1021/ja512868a). The purity of the Cu-NHC catalysts is sufficient to allow direct 'dispensing' of the catalyst solution to catalytic reactions, when required, with no detectable change in catalytic activity and selectivity compared to using the purified catalysts. This will enable rapid catalytic screening, as well as catalyst top-up (coupled with computer controlled reactors) in the near future.
Through collaborations with other research groups in fluid dynamics and inorganic chemistry, we developed an extremely efficient and scalable electrochemical flow-cell which can effect the conversion of imidazoliums and a copper electrode to Cu-NHC catalysts (NHC = N-heterocyclic carbene) in very high yields (DOI: 10.1021/ja512868a). The purity of the Cu-NHC catalysts is sufficient to allow direct 'dispensing' of the catalyst solution to catalytic reactions, when required, with no detectable change in catalytic activity and selectivity compared to using the purified catalysts. This will enable rapid catalytic screening, as well as catalyst top-up (coupled with computer controlled reactors) in the near future.
The technology can be applied to a wide range of ligands beyond NHCs.
CO2 activation and utilisation
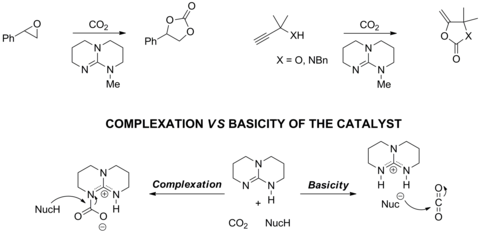 Research in the group investigated the 'activation' of CO2 using guanidine/amidines catalysts and the subsequent catalytic reactions between CO2 and propargylamines. Factors controlling the complexation between amines and CO2 and catalytic activity have been carefully examined using a range of physical organic chemical techniques. This had led to a 10 times increase in the performance of the catalytic system at low loading using a much cheaper catalyst (doi: 10.1039/C4CY00480A and doi: 10.1021/acscatal.7b04108).
Research in the group investigated the 'activation' of CO2 using guanidine/amidines catalysts and the subsequent catalytic reactions between CO2 and propargylamines. Factors controlling the complexation between amines and CO2 and catalytic activity have been carefully examined using a range of physical organic chemical techniques. This had led to a 10 times increase in the performance of the catalytic system at low loading using a much cheaper catalyst (doi: 10.1039/C4CY00480A and doi: 10.1021/acscatal.7b04108).
